Renewable Polyurethanes
We have developed bio-based PU monomers made from algae oils and other biologically-sourced materials and formulated these polyols into PU foams that meet commercial specifications for example for surfboards, shoe midsoles and Flip Flops. We analyzed their biodegradation in compost and soil environments and were able to demonstrate rapid biodegradation through loss of mass and reduced compression force deflection in both environments (see image on the right), as well as identify the consortia of bacteria and fungi associated with the degrading PU foams through metagenomic sequencing. We also cloned and expressed esterase from this metagenome that could degrade the PU foams in vitro back into the starting monomers. These data demonstrated that we can develop rapidly biodegrading PUs that meet the physical specification for real world products and use enzymatic cleavage to recycle those monomers into new PU products.

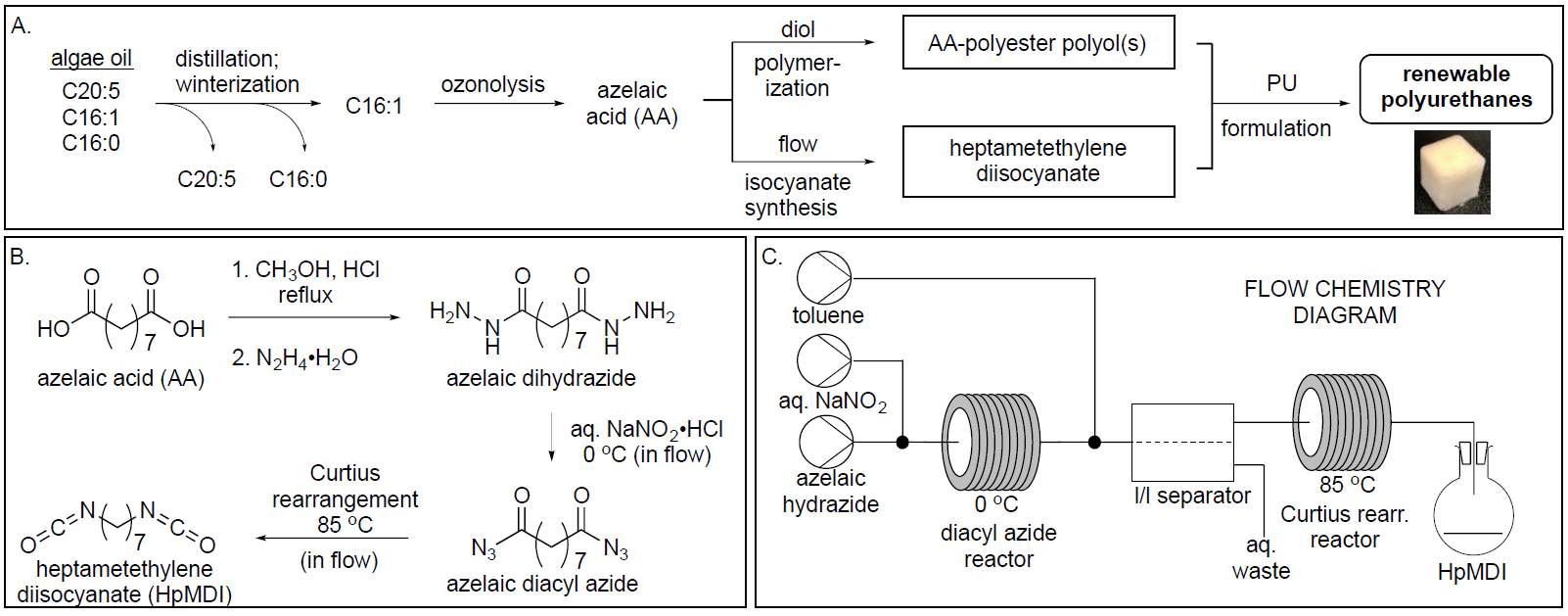
Our most recent efforts in developing PU precursors from algae biomass has identified monounsaturated fatty acids as valuable precursors. Many species of microalgae produce polyunsaturated fatty acids (PUFAs) that have inherent value as omega-3 fats for pharmaceutical and neutraceutical markets, yet these represent only a portion (10-40%) of the total lipids in these organisms, and the other oils are either burned or discarded. These residual fatty acids include primarily palmitic acid (C16:0) and palmitoleic acid (C16:1). We recently developed a methodology for isolating palmitoleic acid from this waste stream (panel A in the figure below). We converted algae-sourced palmitoleic acid into azelaic acid (AA), a 9-carbon diacid, using ozonolysis. with ozonolysis. AA can serve as a precursor for production of PU products, including flexible and rigid PU foams, resins, and coatings. AA can be incorporated into a linear polyester by condensation with a diol, such as renewable propane diol (PDO).
Diisocyanates, the crosslinking side of PU formulations, are currently not commercially available from renewable sources. We recently developed a new method to convert diacids, including AA, into diisocyanates utilizing flow chemistry for a Curtius rearrangement (panels B-C below). This avoids isolating the explosive acyl azide intermediate, thus mitigating safety concerns. Using continuous flow, we prepared acyl azides from corresponding hydrazides and subsequently heated them to undergo Curtius rearrangement, affording diisocyanates in one scalable process. The method is efficient, safe, and scalable, offering a new route to renewable diisocyanates.
 Our most recent efforts in developing PU precursors from algae biomass has identified monounsaturated fatty acids as valuable precursors. Many species of microalgae produce polyunsaturated fatty acids (PUFAs) that have inherent value as omega-3 fats for pharmaceutical and neutraceutical markets, yet these represent only a portion (10-40%) of the total lipids in these organisms, and the other oils are either burned or discarded. These residual fatty acids include primarily palmitic acid (C16:0) and palmitoleic acid (C16:1). We recently developed a methodology for isolating palmitoleic acid from this waste stream (panel A in the figure below). We converted algae-sourced palmitoleic acid into azelaic acid (AA), a 9-carbon diacid, using ozonolysis. with ozonolysis. AA can serve as a precursor for production of PU products, including flexible and rigid PU foams, resins, and coatings. AA can be incorporated into a linear polyester by condensation with a diol, such as renewable propane diol (PDO).
Our most recent efforts in developing PU precursors from algae biomass has identified monounsaturated fatty acids as valuable precursors. Many species of microalgae produce polyunsaturated fatty acids (PUFAs) that have inherent value as omega-3 fats for pharmaceutical and neutraceutical markets, yet these represent only a portion (10-40%) of the total lipids in these organisms, and the other oils are either burned or discarded. These residual fatty acids include primarily palmitic acid (C16:0) and palmitoleic acid (C16:1). We recently developed a methodology for isolating palmitoleic acid from this waste stream (panel A in the figure below). We converted algae-sourced palmitoleic acid into azelaic acid (AA), a 9-carbon diacid, using ozonolysis. with ozonolysis. AA can serve as a precursor for production of PU products, including flexible and rigid PU foams, resins, and coatings. AA can be incorporated into a linear polyester by condensation with a diol, such as renewable propane diol (PDO).